Darkover
Archangel
- Jul 29, 2021
- 5,649
78 percent
The air in Earth's atmosphere is made up of approximately 78 percent nitrogen and 21 percent oxygen. Air also has small amounts of other gases, too, such as carbon dioxide, neon, and hydrogen.
making your own nitrogen at home
Burn a candle in a closed chamber filled with air. When the candle goes out, separate the gas and extract it with water. The CO2 will dissolve in the water, and the remaining gas will be almost pure nitrogen.
This will not work terribly well. Active burning requires some minimum level of oxygen. Maybe as much as 15% in the case of a candle flame, 10–11% for methane or propane. You can certainly *reduce* the oxygen level; but some will likely remain, even if you try to work around the problem by using something like glowing coals, where the heat will drive the reaction even after it's no longer self-sustaining. Not only that, but this kind of reaction will tend to produce soot and carbon monoxide once the oxygen levels get low, which will give you additional problems with scrubbing impurities.
You would be better served by bubbling air through a solution (or series of solutions) that remove the oxygen and carbon dioxide. Oxygen will be taken up by a solution of cuprous chloride (actually, cuprous chloride plus sodium chloride for increased solubility). Carbon dioxide can be removed by a solution of strong hydroxide base, or even sodium carbonate. There are other solutions that would also take up oxygen (ascorbic acid, ferrous salts) but they are less aggressive than cuprous chloride, and might require a tall vessel or multiple passes.
This would still leave inert gases like argon as a minor impurity, but there is no way to 'easily' separate these from nitrogen, and for almost all purposes this doesn't matter.
Generally, industrial production of pure liquid nitrogen is achieved by distilling it from liquid air. Air is composed of oxygen (21%), nitrogen (78%), and a small percentage of other gases.

 en.wikipedia.org
en.wikipedia.org
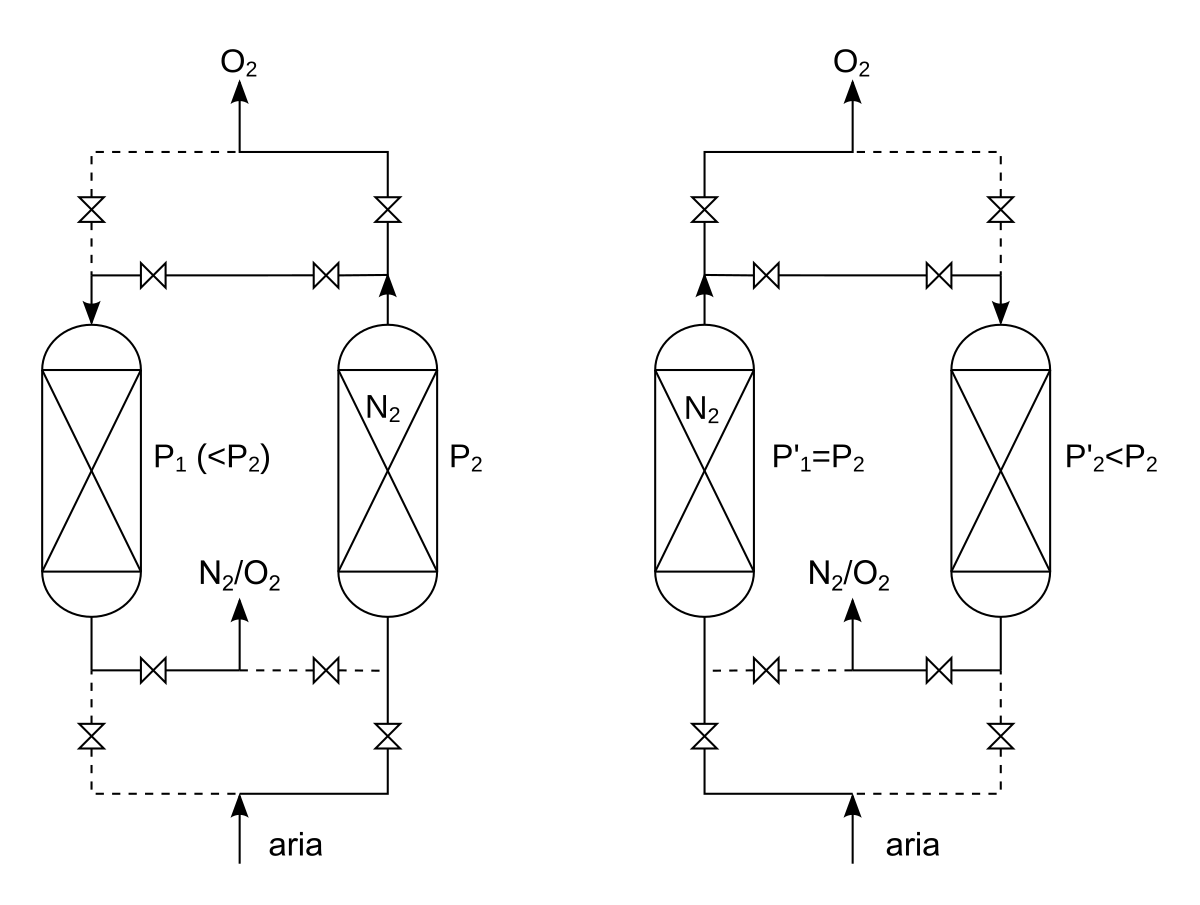
the pressure swing adsorption (PSA) process is based on the phenomenon that under high pressure, gases tend to be trapped onto solid surfaces, i.e. to be "adsorbed". The higher the pressure, the more gas is adsorbed. When the pressure is dropped, the gas is released, or desorbed. PSA can be used to separate gases in a mixture because different gases are adsorbed onto a given solid surface more or less strongly. For example, if a gas mixture such as air is passed under pressure through a vessel containing an adsorbent bed of zeolite that attracts nitrogen more strongly than oxygen, a fraction of nitrogen will stay in the bed, and the gas exiting the vessel will be richer in oxygen than the mixture entering. When the bed reaches the limit of its capacity to adsorb nitrogen, it can be regenerated by decreasing the pressure, thus releasing the adsorbed nitrogen. It is then ready for another cycle of producing oxygen-enriched air.




 nitrogen-generators.com
nitrogen-generators.com
The air in Earth's atmosphere is made up of approximately 78 percent nitrogen and 21 percent oxygen. Air also has small amounts of other gases, too, such as carbon dioxide, neon, and hydrogen.
making your own nitrogen at home
Burn a candle in a closed chamber filled with air. When the candle goes out, separate the gas and extract it with water. The CO2 will dissolve in the water, and the remaining gas will be almost pure nitrogen.
This will not work terribly well. Active burning requires some minimum level of oxygen. Maybe as much as 15% in the case of a candle flame, 10–11% for methane or propane. You can certainly *reduce* the oxygen level; but some will likely remain, even if you try to work around the problem by using something like glowing coals, where the heat will drive the reaction even after it's no longer self-sustaining. Not only that, but this kind of reaction will tend to produce soot and carbon monoxide once the oxygen levels get low, which will give you additional problems with scrubbing impurities.
You would be better served by bubbling air through a solution (or series of solutions) that remove the oxygen and carbon dioxide. Oxygen will be taken up by a solution of cuprous chloride (actually, cuprous chloride plus sodium chloride for increased solubility). Carbon dioxide can be removed by a solution of strong hydroxide base, or even sodium carbonate. There are other solutions that would also take up oxygen (ascorbic acid, ferrous salts) but they are less aggressive than cuprous chloride, and might require a tall vessel or multiple passes.
This would still leave inert gases like argon as a minor impurity, but there is no way to 'easily' separate these from nitrogen, and for almost all purposes this doesn't matter.
Generally, industrial production of pure liquid nitrogen is achieved by distilling it from liquid air. Air is composed of oxygen (21%), nitrogen (78%), and a small percentage of other gases.


Pressure swing adsorption - Wikipedia
the pressure swing adsorption (PSA) process is based on the phenomenon that under high pressure, gases tend to be trapped onto solid surfaces, i.e. to be "adsorbed". The higher the pressure, the more gas is adsorbed. When the pressure is dropped, the gas is released, or desorbed. PSA can be used to separate gases in a mixture because different gases are adsorbed onto a given solid surface more or less strongly. For example, if a gas mixture such as air is passed under pressure through a vessel containing an adsorbent bed of zeolite that attracts nitrogen more strongly than oxygen, a fraction of nitrogen will stay in the bed, and the gas exiting the vessel will be richer in oxygen than the mixture entering. When the bed reaches the limit of its capacity to adsorb nitrogen, it can be regenerated by decreasing the pressure, thus releasing the adsorbed nitrogen. It is then ready for another cycle of producing oxygen-enriched air.




On-Site Nitrogen Production - Nitrogen Generators | CGT
There are two common methods for On-Site Nitrogen Production, membrane and PSA (Pressure Swing Adsorption). This article will explain how each methods works
Last edited: